-40%
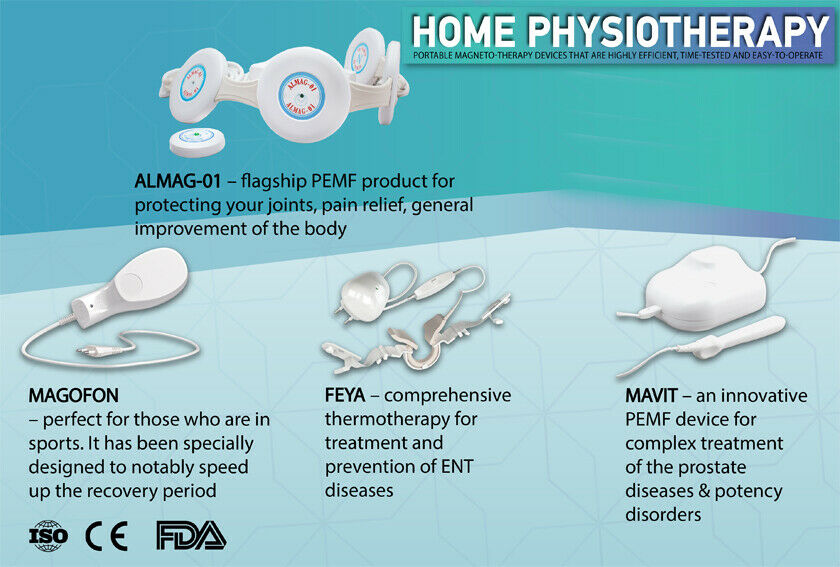
ALMAG-01 portable magnettic therapy Device 220v/50Hz, Modell 2020
€ 180.04
- Description
- Size Guide
Description
ALMAG-01Attention devices Almag-01 in light blue Box may not be used in EU countries.
Care before forgeries!
1. SPECIFICATIONS
1.1 Mains circuit voltage: ~220V (-10%; +10%) or ~230V (-10%;+6%), frequency 50Hz ,
1.2 Power consumption: not more than 35 VA.
1.3 Weight: max. 0,72 kg.
1.4 Overall dimensions of: electronic unit 137x60x44 mm one of the coils of exposure assembly - 15xǾ90 mm Note: extreme deviations: ±3%.
1.5 The number of exposing coils-inductors - 4.
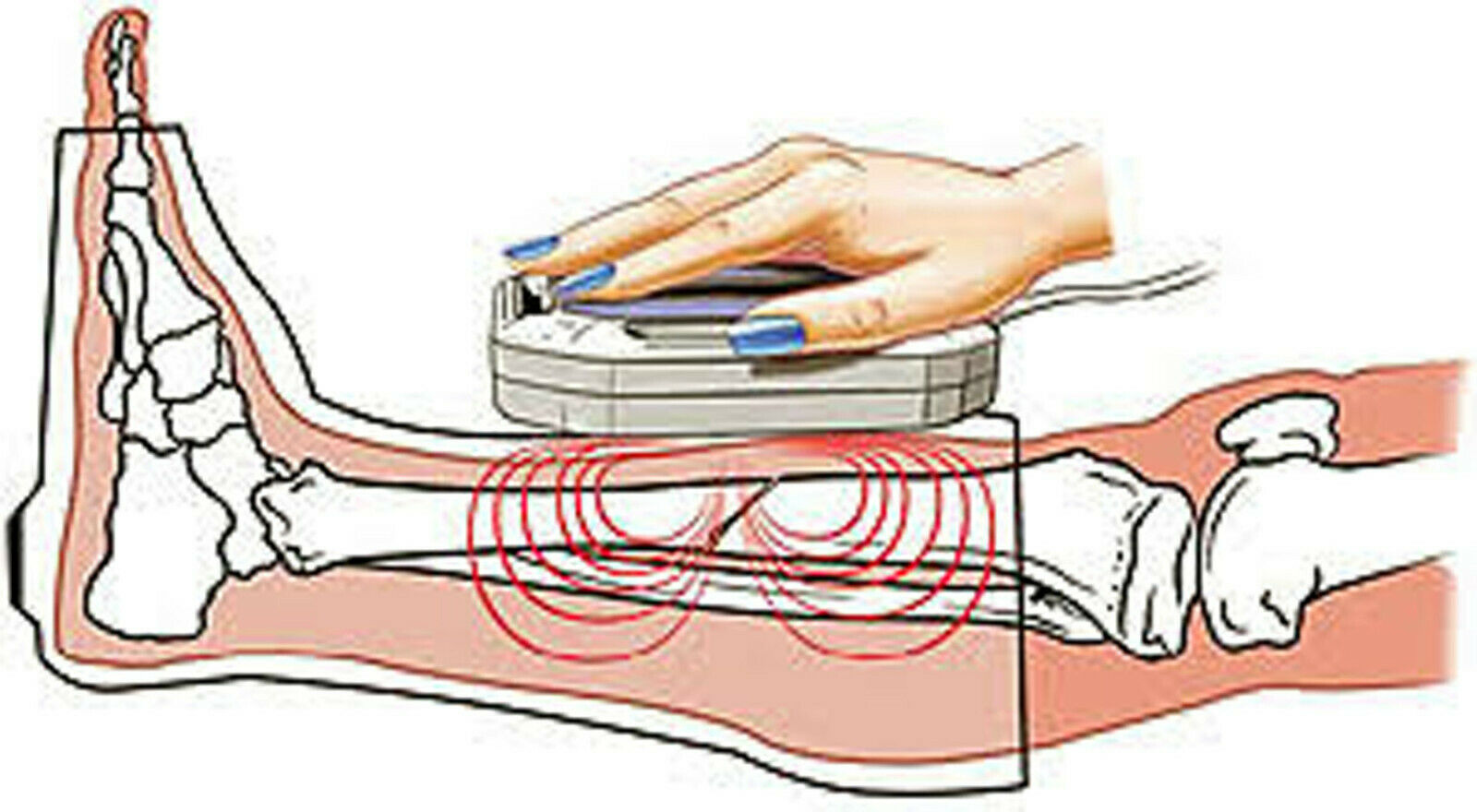
1.6 Amplitude value of magnetic induction on the functional surface (both flat surface) of the device exposure assembly coil-indictor: (20±6) mTl.
1.7 Pulse duration: 1,5 - 2,5 ms. Magnetic field pulse repetition frequency per coil: 6 Hz.
1.8 The device activation, its connection to the mains, is followed with LED light indication.
1.9 The device provides recurring-short-term operating mode within 6 hours: operation (action) period - (22±1) min., break - 10 min.
1.10 Device is automatically disconnected from the electric mains after (22±1) min. operation.
1.11 Environmental resistance of the Device is the type of moderate cold climate design apparatus.
1.12 The outer surfaces of the device are resistant to disinfection by chamical method: 3% solution of hydrogen peroxide with 0,5% detergent of “Lotos” type or 1% solution of chloramine.
2.13 Mean-time-between-failure: min. 1000 h.
2.14 Mean service life - five years.
2.15 The Device is made of the material which produces no irritating or allergic effect.
2.16 Maximum temperature after one operation cycle: - device case, max. - 45 °C; - device exposure assembly unit , max. – 41 °C 2.17 Class of the device depending on potential risk of application - 2а.
ALMAG-01
Operating manual
3. DELIVERY COMPLETE SET The delivery complete set includes:
- "ALMAG-01" device;
- magnetic field indicator;
- operating Manual.
PURPOSE OF THE DEVICE
1.1 General information
1.1.1 The Device is designed to produce a therapeutic effect on the human body with a travelling pulsed magnetic field and can be applied both at physiotherapeutic departments of patient care and prophylactic institutions and by a patient himself at home conditions.
1.1.2 The Device is to be sold either through retail or wholesale trading network..
1.1.3 The Device is designed to operate under normal environmental conditions for Temperate Cold climate design products, ambient temperature between +10°C - +35°C, atmospheric pressure from 86,6 kPa to 106,7 kPa (650 - 600 mmHg).
1.1.4 Electric safety class of the Device: Class II of B type according to IEC 60601-1-2.
1.2 Indications for application
Locomotor system diseases:
- spinal osteochondrosis with reflex cervical spine, thorax and loin radicular syndrome
- deforming osteoarthrosis
- arthritis and arthrosis of different joints: scapulohumeral periarthrosis, arthritis, epicondylitis, gout
- bursitis
- myositis
- parantnoit Locomotor system injuries and their after-effects:
- bone fractures; - internal joint damages; - posttraumatic joint contracture;
- wound, soft tissue bruise, hematoma, posttraumatic edema;
- muscle and ligaments injuries;
- postoperative wounds;
- sluggish purulent wounds, phlegmons, burns. Diseases of peripheral nervous system:
- neuritises: - facial nerve neuritis
- radial nerve neuritis
- ulnar nerve neuritis
- median nerve neuritis
- sciatic nerve (ischias) neuritis
- peroneal nerve neuritis
- plexitis
- neuralgias:
- trigeminal nerve neuralgia,
- occipital nerve neuralgia,
- intercostal neuralgia Traumas of central nervous system:
- trauma of a backbone and a spinal cord;
- disorder of a spinal blood circulation, Venous disorders of upper and lower extremities:
- thrombosis of deep crus veins;
- chronic thrombophlebitis at the trophic disturbance stage;
- varicose disease
1.3. Contraindications:
- inflammatory diseases in the acute phase;
- hemorrhage and predisposition to it;
- high-grade hypotension;
- purulent processes before surgical treatment;
- severe cardiac ischemia;
- early post infarction period;
- acute phase of cerebral blood circulation disturbance (stroke);
- pregnancy;
- systemic blood diseases;
- oncological diseases;
- thyrotoxicosis;
- diencephalic syndrome;
- the presence of implanted cardiostimulator in the zone of action